The porcine sciatica model can evaluate efficacy and PK/PD from the same subject and may provide more clinically relevant data at preclinical stages minimizing expensive failures.
MD Biosciences has developed a large animal model of neuropathic pain for preclinical evaluation of analgesic or neuroprotective therapies. The sciatica model was developed in the pig, as the innervation is similar to that of humans, providing more clinically relevant data at the preclinical stages. The model is suitable for evaluating both efficacy and PK/PD in the same animal and can be used for systemic, local and cell therapies as well as slow release drugs and devices.
Creating injury directly on the sciatic nerve by either nerve crush or nerve cutting induces neuropathic pain and minimizes the inflammatory component. The nerve crush method produces a less pronounced pain as well as a quicker recovery for the subjects. For therapies that are thought to be neuroprotective or promote nerve regeneration, partial nerve damage using the nerve crush method will allow direct treatment to the nerve. Using either method, subjects are completely recovered by 7 days post surgery and can be evaluated for pain up to 21 days. In addition to sensitivity to pain and pain sensation, subjects avoid using the ipsilateral leg suggesting a responsiveness to weight bearing and sustained pain.
Readouts:
- Von Frey
- Cold allodynia
- Pin prick
- Weight bearing
- Histology
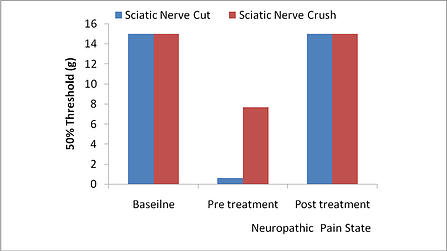
Figure: Subjects underwent either nerve crush or nerve cut methods. Subjects were tested for sensitivity to Von Frey pre- and post-treatment with 1 mg/kg morphine. Data is reported using Von Frey method and shows the 50% threshold in grams (g).
The porcine sciatica model of neuropathic pain is just one of many large preclinical models offered by MD Biosciences. With the high failure rate at clinical trial phases, more relevant preclinical models are needed to assess candidates at earlier stages. The pig is an ideal model system due to its human similarities in innervations, skin and the cardiovascular system. Other porcine models offered by MD Biosciences include post-operative pain, myocardial infarct, diabetes and diabetic neuropathy.
About MD Biosciences
MD Biosciences is a Preclinical and Clinical Contract Research Organization (CRO) providing services for biotech/pharmaceutical, medical device and animal health. Our core therapeutic focus is in inflammation/autoimmune, neurology/CNS disorders, pain and cardiovascular as well as the interplay between the inflammatory, neurology and cardiovascular systems. Our approach is to work backwards from the clinic, understanding what the clinicians would expect from an active compound. This approach and understanding enables the development of models and biomarkers that help break the preclinical/clinical barrier and provide more clinically relevant data at earlier stages.
--------
The information in this press release should be considered accurate only as of the date of the release. MDB has no intention of updating and specifically disclaims any duty to update the information in these press releases.