MD Biosciences focuses on rapid and cost effective models of psoriasis involving the IL-23/TH17 axis in order to provide companies alternatives to complex and costly xenotransplantation models for screening. With the earlier launch of the IMQ-induced model and now the IL-23 psoriasis model, MD Biosciences offers a range of rapid models for dermal conditions such as dermatitis and psoriasis.
Recent psoriasis research has focused extensively on the IL-23/TH17 axis. While previously thought to be mainly a Th1 mediated disease, evidence has shifted to a new population of IL-17 producing Th17 cells and the involvement in a variety of autoimmune diseases including psoriasis. The development and maintenance of Th17 cells has been linked to IL-23, a key cytokine involved in the development of autoimmunity. With the focus of research on the IL-23/TH17 role in psoriasis, a number of novel therapeutics are currently in clinical trials that target this pathway.
MD Biosciences is currently working with a biopharmaceutical company in evaluating novel therapeutics that target this pathway. While models of psoriasis such as xenotransplantation models have traditionally been the most commonly used, they are complex and cost prohibitive for screening. MD Biosciences research group has validated rapid and cost effective preclinical models for screening psoriatic therapeutics. In addition to the IMQ-induced psoriasis model launched last year, the most recent model to be validated is the IL-23 induced model of psoriasisform inflammation.
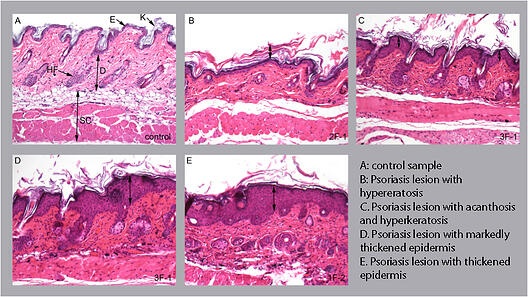
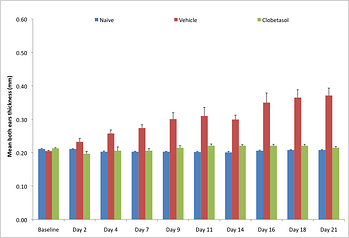 The IL-23 psoriasis model is 21 days long and involves the injection of IL-23 into the ear of C57Bl/6, which produces psoriasis-like inflammation that is dependent on IL-22. IL-22 induces keratinocyte proliferation and epidermal hyperplasia contributing to epidermal thickening. “We are continually looking to the most recent research clinical findings. With the number of clinical trials involving targets in the IL-23/Th17 pathway, offering rapid preclinical models of psoriasis involving this pathway allows biopharma companies to cost-effectively screen potentially novel therapeutics. This is important for companies in early discovery to be able to evaluate compounds in a clinically relevant model of psoriasisform inflammation.”
The IL-23 psoriasis model is 21 days long and involves the injection of IL-23 into the ear of C57Bl/6, which produces psoriasis-like inflammation that is dependent on IL-22. IL-22 induces keratinocyte proliferation and epidermal hyperplasia contributing to epidermal thickening. “We are continually looking to the most recent research clinical findings. With the number of clinical trials involving targets in the IL-23/Th17 pathway, offering rapid preclinical models of psoriasis involving this pathway allows biopharma companies to cost-effectively screen potentially novel therapeutics. This is important for companies in early discovery to be able to evaluate compounds in a clinically relevant model of psoriasisform inflammation.”
Background on Th17 in psoriasis pathology
While the pathology of psoriasis is not completely understood, there are complex un- derlying mechanisms, which involve the interplay between epidermal keratinocytes, leukocytes such as dendritic cells and APCs, and vascular endothelium. Epidermal keratinocytes and vascular endothelial cells are active participants in the psoriasis inflammatory process via secreted cytokines and growth factors along with the upregulation of signaling and adhesion molecules on their surfaces. TGFb1 is elevated and released by keratinocytes, which when combined with activated dendritic cells is sufficient to generate TH17 cells in skin-draining lymph nodes inducing cutaneous inflammation. Intracellular signaling induces expression of IL-23R on developing Th17 cells promoting responsiveness to IL-23, the key cytokine in the survival and proliferation of Th17 cells. Th17 cells secrete a range of pro-inflammatory cytokines such as IL-6, IL-17A, IL-17F, IL-21, IL-22 and TNF-α . In psoriasis, IL-23 plays an important role with genetic alterations of IL-23p40 and IL-23R leading to enhanced IL-23 production increasing psoriasis susceptibility and indeed injection of the IL-17 supporting cytokine, IL-23, into the skin of mice can induce psoriasis-like inflammation. The main cellular source of IL-17A in psoriasis-like skin inflammation are γδ T cells located specifically in the dermal layers and expansion of Th17 cells producing inflammation and these cells are required to initiate the IL-23 driven processes.
About MD Biosciences
MD Biosciences is a Research Group and Preclinical Contract Research Organization (CRO) providing services and products for biotech/pharmaceutical, medical device and animal health and companies engaged in inflammations & neurology research. With specialized laboratories located in Minnesota, Glasgow, and Israel, our panel of internationally recognized experts provides in-depth expertise and technologies to overcome challenges and provide total solutions to the drug discovery market.
--------
The information in this press release should be considered accurate only as of the date of the release. MDB has no intention of updating and specifically disclaims any duty to update the information in these press releases.