Unlocking value early in drug development and increasing the translational power of preclinical studies.
The use of biomarkers has the potential to increase value early in drug development by extracting more data and information from each study. This can increase efficiency, reduce costs, guide decisions, boost the translational power of preclinical studies, and effectively enable more drug candidates to move forward.
MD Biosciences has expertise with biomarker analysis for markers that play a role in neuroinflammation, neurodegeneration, and pain. Examples of methods we incorporate are listed below.
Preclinical and Clinical Biomarker Assays
-
High Throughput Screening Assays
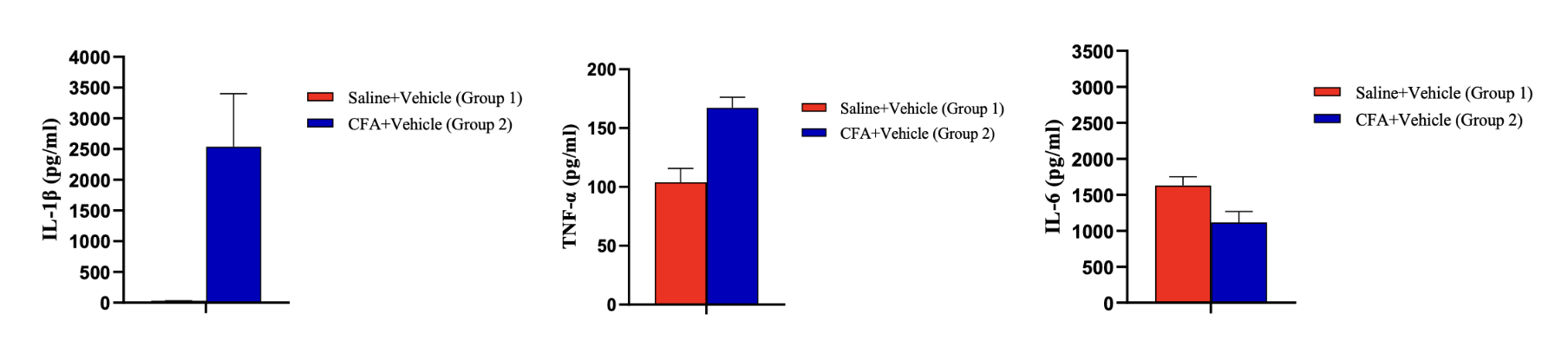
Multiplex immunoassays have enabled screening of important biomarkers involved in disease modulation and progression. Multiplexing makes it possible to measure up to 50 biomarkers simultaneously from a small sample size, which is a crucial advantage when dealing with limited sample volume from preclinical and clinical studies. By analyzing multiple biomarkers related to pain and neurological conditions, researchers can detect changes in individual cytokines, and identify unique trends associated with early disease progression and mechanism of action. This tool helps explain the crosstalk between the nervous system and inflammatory system and highlights specific targets that require greater levels of sensitivity. Even though high throughput assays are a robust screening tool, neuroscience-specific analytes can remain undetected and high sensitivity target analysis is needed as a next step.
High-Throughput Screening Ultra-sensitive Detection Sensitivity pg/mL fg/mL Sample Volume < 25 uL 5-100 uL Dynamic Range 3-5.5 log 3-5.5 log Sample Type CSF, plasma, serum, tissue CSF, plasma, serum, tissue Species human, rodent, pig, other human, rodent, pig, other Sample data: Pro-inflammatory and anti-inflammatory cytokines in rat paw skin. IL-1β and TNF-α increase significantly, showing an inflammatory response in the tissue.
-
High Sensitivity Femtogram Assays
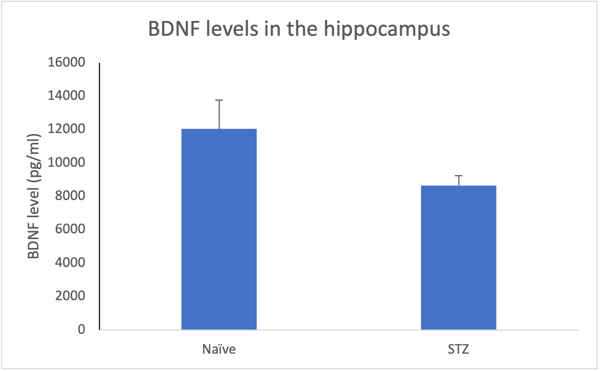
High sensitivity biomarker evaluation for clinical and preclinical research is a non-invasive platform that can detect those difficult to measure analytes. This method takes multiplexing further into a specific target with fewer limitations. Traditional assays depend on invasive CSF sampling and cannot detect low abundant markers in blood-based samples, which is the ideal sample type for biomarker analysis. In general, 75% of proteins are at low abundance and cannot be detected, and 25% of proteins remain undetectable at a healthy state. High precision single plex digitally quantifies low abundant proteins, and is reliable, accurate, reproducible, and highly sensitive. This platform has better precision, a large dynamic range, and requires smaller sample volume, which leads to clinical relevance and accuracy.
High-Throughput Screening Ultra-sensitive Detection Sensitivity pg/mL fg/mL Sample Volume < 25 uL 5-100 uL Dynamic Range 3-5.5 log 3-5.5 log Sample Type CSF, plasma, serum, tissue CSF, plasma, serum, tissue Species human, rodent, pig, other human, rodent, pig, other Sample data:
BDNF levels in the hippocampus 28 days following STZ. Decreased levels may show the beginning of degradation in diabetic neuropathy.
-
Ultra Sensitive NFL Detection
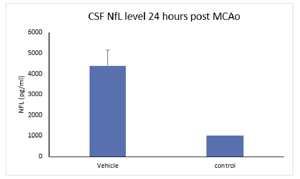
Neurofilament light (NFL) is a protein expressed in myelinated axons that reflects the level of axonal damage in the nervous system. NFL is released into the CSF or blood following axonal damage and is an indicator of disease progression. NFL is typically elevated in neurodegenerative or neuropathic pain conditions.
High-Throughput Screening Ultra-sensitive Detection Sensitivity pg/mL fg/mL Sample Volume < 25 uL 5-100 uL Dynamic Range 3-5.5 log 3-5.5 log Sample Type CSF, plasma, serum, tissue CSF, plasma, serum, tissue Species human, rodent, pig, other human, rodent, pig, other Sample data: NfL levels in rat CSF and blood following MCAO in rats. NfL levels were elevated in MCAO treated animals compared to control or naïve animals 24 hours following occlusion.

-
ELISA
ELISA immunoassays are plate-based assays that detect and quantify target proteins. This tool offers high sensitivity and specificity for one analyte per run, while the multiplex assay can measure several markers per run.
-
Western Blot
MD Biosciences runs Western Blot services for target protein detection. Western Blot is an accurate and quantitative assay for analyzing protein expression in cells.
-
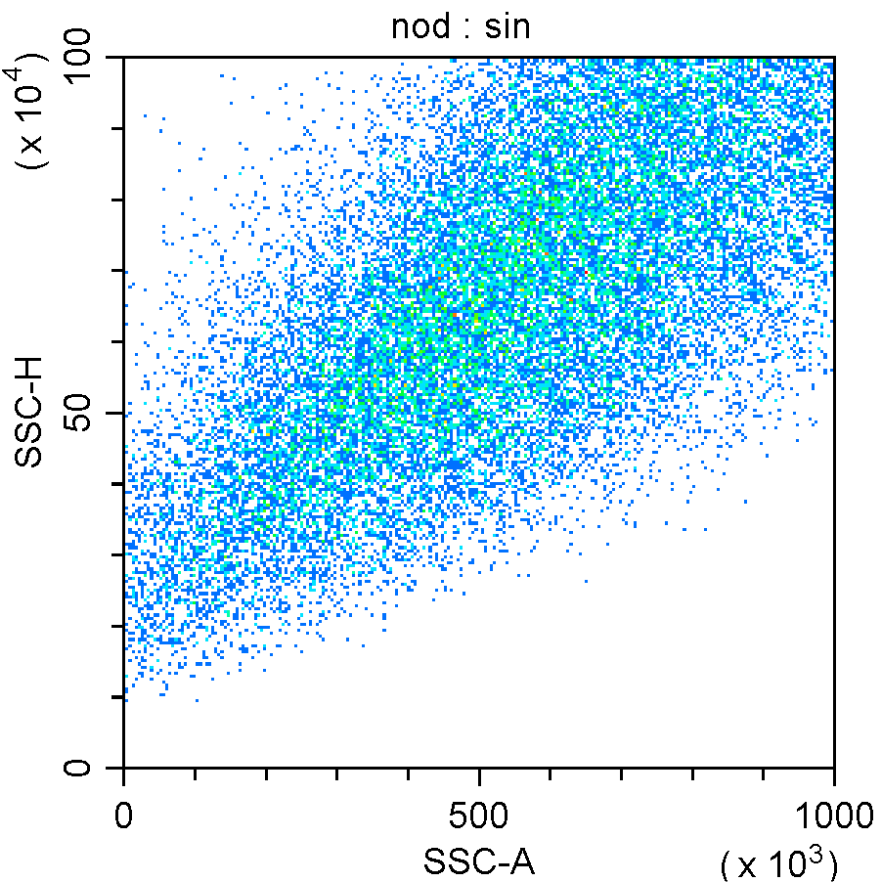
Flow Cytometry
MD Biosciences offers immunophenotyping by flow cytometry to assess efficacy, PK/PD and mechanism of action. Flow cytometry is the recommended analytical method to identify cell populations in blood and tissues.
Our scientists can help design relevant panels that are either pre-validated or custom validated for preclinical and clinical evaluation.
Click here for more information on histology services.
Biomarker Resources
Whitepaper: Translational Biomarkers
This whitepaper provides an overview of how biomarkers can be utilized to extract maximum value early in translational studies while being optimized for use in clinical studies.
Whitepaper: Inflammatory Biomarkers
The interplay between the central and peripheral nervous systems and the immune response highlights the importance of understanding the cellular and inflammatory attributes that are strongly associated with neurological diseases and pain.
Whitepaper: High Precision Biomarker Detection
Multiplex and ultra-sensitive detection assays provide a powerful platform for difficult to measure analytes. The detection of these markers offers invaluable insight on monitoring disease progression at earlier stages and assessing the need for therapeutic intervention.
Ready to discuss your project?
If you are ready to discuss how electrophysiology can add to your understanding of your therapeutic potential, our scientists are eager to explore the possibilities with you. Like many other pharmaceutical and medical device developers, you can rely on predictive preclinical data.



