The problem: the rat model is limited in assessing any local treatment (cream, skin injections, patches and nerve blocks).
The solution: translational models in pigs, prior to clinical stages.
- A cutting-edge translational model (technically and scientifically): volume of injection, nerve stimulation, imaging, PK, behavioral changes and skin biomarkers.
- By utilizing more translational models in larger species, the success rate of drugs entering clinical phases is dramatically increased.
Now offering a unique model allowing the differentiation between motor dysfunction and pain block following drug application.
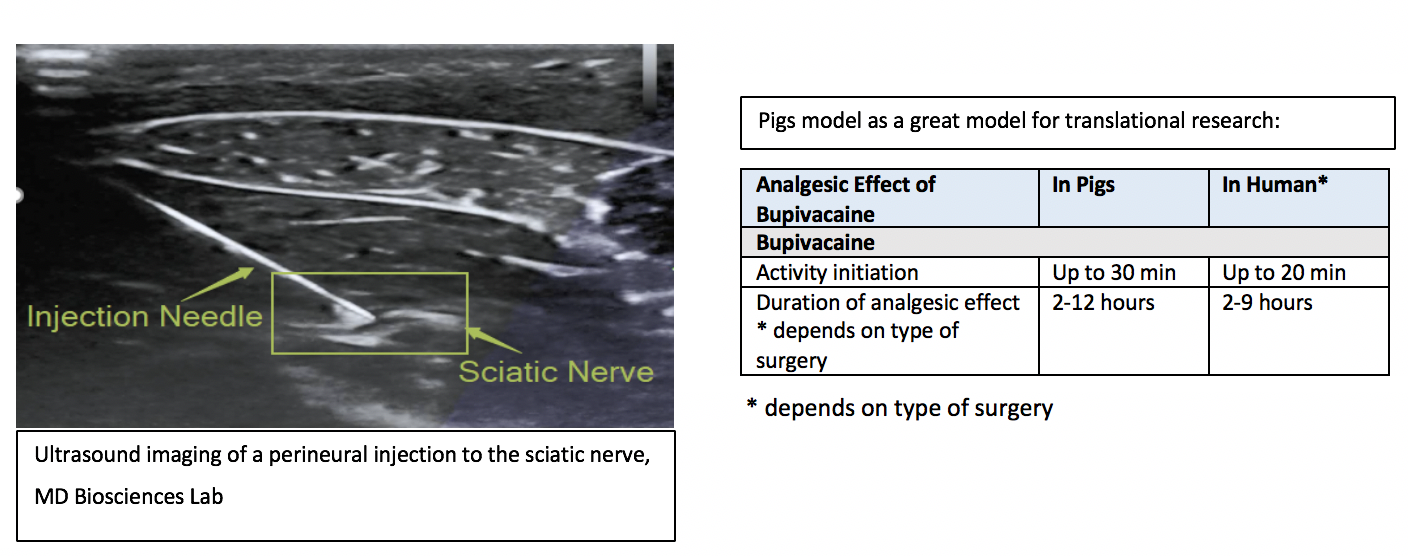
Unique Model Characteristics
- Similar to the clinic - ultrasound guide and nerve stimulation dosing
- The study enables the differentiation between motor dysfunction and pain block following drug administration
- Various readouts: von Frey-stimulation (primary pain), behavior score, motor function score, open field test, incision score, incision healing (histology) and biomarker analysis in skin
We also offer a wide variety of rodent models and services across therapeutic areas including inflammation, pain, neurodegenerative disease, wound healing, histology, cell-based assays, biomarker analysis and statistical review and more to compliment your studies. We look forward to learning more about your research needs and working together. Call us today: 651-641-1770.








