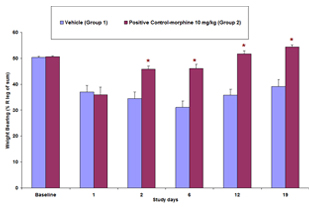
Osteoarthritis (OA) is a widespread condition that affects greater than 70% of the elderly population and poses a heavy cost burden on healthcare. It is a chronic degenerative disease characterized byt the loss of articular cartilage components, which affects the entire joint structure. One of the major complaints by OA patients is the loss of joint function as well as chronic pain. Current therapies are focused on alleviating joint pain, however full pain relief is rarely experienced and significant side affects are commonly present. Research is not only focused disease pathology but also on understanding the mechanisms responsible for induction and maintenance of pain states.