We are happy to announce that we have established MD Biosciences Neuro, LLC. in Cambridge, Massachusetts to expand our work and collaborations in the United States. Our team, led by Chief Scientific Officer, Sigal Meilin, is looking forward to developing close partnerships with you and continuing to advance the field of neurology and pain research.
 With a multifaceted approach to assessment of neurological diseases, specializations include pain-related work, neurodegenerative diseases including Parkinson's, neuro-inflammatory diseases such as Multiple Sclerosis (MS), and capabilities in stroke and cerebral ischemia. Preclinical models are complimented by in house histology, biomarker analysis and electrophysiology capabilities. Finally, our unique porcine models share a high degree of similarity to humans, thus offering greater clinical efficacy that serve to augment and accelerate your program towards their next objectives and milestones.
With a multifaceted approach to assessment of neurological diseases, specializations include pain-related work, neurodegenerative diseases including Parkinson's, neuro-inflammatory diseases such as Multiple Sclerosis (MS), and capabilities in stroke and cerebral ischemia. Preclinical models are complimented by in house histology, biomarker analysis and electrophysiology capabilities. Finally, our unique porcine models share a high degree of similarity to humans, thus offering greater clinical efficacy that serve to augment and accelerate your program towards their next objectives and milestones.
Please contact us with any questions about our services, capabilities, and customizable study design options.

Read More
Topics:
Pain,
news,
Neuro/CNS,
Preclinical Discovery,
neuropathic pain,
Life Science,
translational,
assays,
drug development,
preclinical research,
disease models,
Biotechnology,
Behavioral Research,
porcine model,
Neuroscience
MD Biosciences lab was featured with Ellegaard offering an exclusive expertise in pain studies. The article discusses our work to develop and validate models in adult pigs, specifically for chronic and acute pain.

When do we recommend to work with minipigs?
MD Biosciences pigs studies include domestic pigs and Göttingen minipigs.
The main advantage of the minipig is that it gains weight much slower than the domestic pig. This could be significant when:
- The study requires working with adult animals
- When we run models with chronic diseases ( ≥3 months)
- Tox and ADME studies are run in Göttingen minipigs, which is significant for standardization
Pig models at MD Biosciences:
Post-Operative Pain
Modified Post-Operative Pain
Neuropathic Pain
Wound Healing
Ready to learn how your research can accelerate with pig studies? Contact us today! +1 (651) 641-1770 or info-us@mdbiosciences.com
Read More
Topics:
Pain,
post-operative pain,
neuropathic pain,
translational,
Wound Healing,
translational research,
Behavioral Research,
porcine model,
Neuroscience
Now live: a website dedicated to MD Biosciences' cutting-edge neurological and CNS research services offered through our Neuroscience Research Laboratories. Our facilities are fitted with state-of-the-art equipment and technologies for preclinical and translational studies, featuring clinically-relevant read outs and endpoints. Specialties include studies of neuropathic, acute and chronic pain and neurodegenerative/neuroinflammatory diseases. Unique pig translational models and electrophysiology capabilities compliment rodent capabilities and accelerate your program towards their next objectives and milestones. Contact our team today! 651-641-1770 or info@mdbiosciences.com

Read More
Topics:
Pain,
news,
Neuro/CNS,
in vivo pain models,
post-operative pain,
Biomarkers,
neuropathic pain,
Neuropathy,
assays,
Batch Release Testing,
preclinical research,
translational research,
Electrophysiology,
Behavioral Research,
porcine model,
Neuroscience,
Nerve Injury
Despite massive investment in research for treatment options, there are not many effective and safe therapeutics for human chronic neuropathic pain (NP) afflictions. Options are limited due to the lack of translatable animal models, especially large animals who have similar physical and metabolic features to humans (Rice et al., 2008, Henze and Urban, 2010, Swindle et al., 2012). MD Biosciences, Inc. has developed a modified unilateral sciatic nerve PNT model in pigs that produces sustained NP behaviors consistent with those observed in human pain patients. The pig PNT model provides highly valuable data for translational pain research.
Read more in our scientists' latest publication here!
MD Biosciences translational models can be enhanced with behavioral analysis capabilities, electrophysiology recordings, histology/IHC services and more. Contact us today for more information: info-us@mdbiosciences.com or (651) 641-1770.

Read More
Topics:
Pain,
neuropathic pain,
translational research,
Behavioral Research,
porcine model,
Histology
We recently published a publication demonstrating the utility of open field testing in combination with behavioral scoring in the pig peripheral neuritis trauma (PNT) model for pain. Combined together and applied in this model, they represent a powerful tool to assess the spontaneous behavior of pigs in response to pain. Rodent models are often used in pain research as they offer valuable data regarding underlying mechanisms contributing to pain, though they are limited in their translatability to human application. A pivotal benefit of using pig models is their increased translatability to the clinic considering the anatomical and physiological similarities pigs share with humans in comparison to rodents. This paper suggests that pig behavioral patterns are similarly translatable. MD Biosciences is proud to be a leader in neurology-related research, offering a variety of pig studies to enhance development in multiple therapeutic areas.
For more information regarding our pig study services, visit our website or contact us!
Read More
Topics:
Pain,
Stress, trauma pain, pain biomarkers, CNS imaging,
Academic Researh,
preclinical research,
translational research,
Behavioral Research,
porcine model
Proud to announce that the pig post operative pain model pioneered by our scientists was used and cited in a clinical study poster publication written by Heron Therapeutics to assess the synergistic effect of Bupivacaine and Meloxicam in HTX-011.
"Meloxicam and Bupivacaine combined in a single extended-release formulation (HTX-011) delivered at the wound site in a preclinical post-surgical pain model in pigs exhibited greater analgesia than either compound delivered alone within the same extended-release formulation; this finding was confirmed in an initial clinical trial in bunionectomy" (Heron Therapeutics, 2018).
To learn more about our rodent and pig post-operative pain models, visit our preclinical pain webpage or view our datasheet below.

Read More
Topics:
Pain,
post-operative pain,
neuropathic pain,
preclinical research
MB Biosciences is proud to announce our newly established electrophysiology capabilities suited for preclinical neuropathy research! Our lab has acquired the cutting-edge Dantec KEYPOINT Focus tool utilized to measure electrophysiological action potentials. Our scientists have applied these new capabilities in the assessment of Muscle Action Potential (measuring signals from the nerve to muscle) in rodent models of peripheral nerve injury. Using these capabilities, we can measure nerve regeneration, degeneration and protection in response to various treatments. This technology can be further applied to non-rodent species such are our pig translational pain models. Additionally, we are now offering measurement capabilities for Motor Action Potential with a specialized Renovo Neural paradigm—particularly useful for studies of EAE, MS, spinal cord injuries and CNS pain. Application of these capabilities specifically to EAE and MS models provide valuable insights into multiple areas of compound efficacy. View the graphs below to learn more about the ways in which using intraoperative techniques applied in the brain, peripheral nerve surgeries and spine has led us to developing models for monitoring impairment in neural activity. By empirically evaluating neural diseases and their physiological symptoms, we can effectively offer precise measurements for drug effect and treatment options.
To learn more about how this technology can be applied to specific study needs, please contact us.
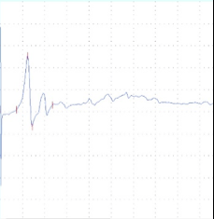
In-vivo neurophysiology
A B C
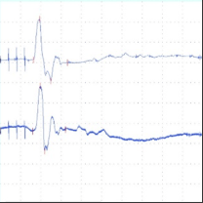
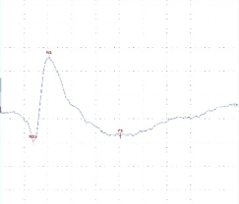
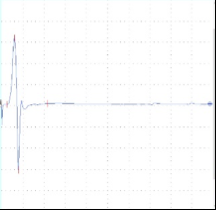
Figure 1. A) CNS model - Transcranial electric motor evoked potentials (tcMEPs) were recorded from the bilateral tibialis anterior (TA) muscles using paired subdermal needle electrodes. TcMEP stimulating electrodes were placed anterior to the C3 and C4 scalp positions. B) Pain and sensory model - Somatosensory evoked potential (SSEP) - electrodes were placed along the medial aspect of each ankle at the malleoli for bipolar stimulation of the posterior tibial nerves (PTN). Somatosensory evoked potentials (SSEP) were recorded over the cerebral cortex using subdermal needle electrodes placed posterior to the C3 and C4 scalp positions. Recording electrodes were referenced to the FPz position on forehead. C) PNS model - Compound muscle action potential (CMAP) - recorded from the tibialis anterior muscle using paired subdermal needle electrodes. Stimulating electrodes were placed along the sciatic notch. Data were recorded from a Sprague Dawley rat.
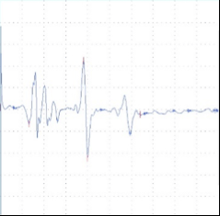
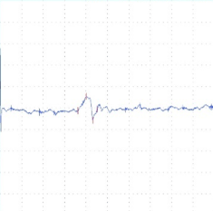
A B C
Figure 2: tcMEP Recordings from TA muscle in a multiple sclerosis model. A A healthy potential recorded from naive mouse (C57). B) Desynchronized potential recorded from a mouse treated with MOG demonstrating a clinical paralysis score of 1-2 (mild paralysis). Note multiple picks of potential, late latency for the main potential and extended duration of the total event. C) Small and weak potential recorded from a mouse treated with MOG demonstrating a clinical paralysis score of 3-4 (moderate paralysis).
Read More
Topics:
Pain,
neuropathic pain,
preclinical research,
Electrophysiology
MD Biosciences will be presenting its newest advances in translational pig models for pain at this year's SMi Pain Therapeutics Conference to be held in London, UK on May 22-23. Translational models in pigs for the study of pain have become an indispensable tool in drug development. The skin structure of the pig, particularly with respect to its neuronal structure, shows a high degree of similarity to that of humans. This provides a platform in which new therapeutics can be tested to yield results which are highly predictive of human outcomes. MD Biosciences has continuously developed its models to approximate the human condition as closely as possible, bringing its unique capabilities to the drug development process. Please contact MD Biosciences or SMi Group Ltd for conference details.
Read More
Topics:
Pain,
in vivo pain models,
post-operative pain
 MD Biosciences article Characterization of a porcine model of post-operative pain (POP) has been published in the European Journal of Pain, Sept 2013. Dr Sigal Meilin, lead neurologist and research director at MD Biosciences, participated in developing a porcine model of post-operative pain that we propose provides greater translational relevance for the evaluation of local treatments of POP compared with existing rodent models of incisional pain.
MD Biosciences article Characterization of a porcine model of post-operative pain (POP) has been published in the European Journal of Pain, Sept 2013. Dr Sigal Meilin, lead neurologist and research director at MD Biosciences, participated in developing a porcine model of post-operative pain that we propose provides greater translational relevance for the evaluation of local treatments of POP compared with existing rodent models of incisional pain.
While the rodent model is commonly used for evaluating POP, one of the major disadvantages is that it is limited in its use in assessing topical and localized treatments such as devices or patches as the hindpaw is relatively small and the rodent may lick or bite at the injured paw. The rodent skin is also very different from human skin in that it heals primarily through contraction rather than re-epithelialization. Pigskin exhibits a higher degree of homology to human skin and has considerable correlation between contractile, metabolic and morphological features in skeletal muscle of human and pig. This model as has three important parameters that can be assessed in parallel:
- Nociceptor sensitivity
- Spontaneous behavior
- Wound healing and inflammation
The following abstract is from the publication in European J Pain, Sept 2013:
Abstract
Background:
Management of acute pain related to surgical intervention, termed post-operative pain or POP, continues to be a major healthcare challenge. While the rat plantar incision model provides valuable data to researchers about the mechanisms mediating POP, the development of topical and localized treatments in small animal models is limited. To help address these issues, we describe here the characterization of a large animal model of incisional pain.
Methods
Pigs underwent full-skin incision or full-skin and muscle incision and retraction (SMIR). Withdrawal thresholds were determined using the Von Frey test at baseline, 0.5–12 h post-surgery and on days 1, 2, 3, 5 and 7 post-surgery. The analgesic effects of systemic morphine [0.1 or 1.0 mg/kg intramuscular (i.m.) dose] and local anaesthetic ropivacaine were studied. Spontaneous pain-like behaviours were scored and analysed. The effects on wound healing were evaluated by gross observation and by histopathological examination.
Results
Pigs incurring SMIR demonstrated significantly increased mechanical hypersensitivity compared with pigs that underwent full-skin incision only (p < 0.05). Maximal analgesia was achieved with morphine (1 mg/kg i.m. dose) at 0.5 h post-treatment. Local treatment with ropivacaine was effective at increasing the withdrawal threshold to Von Frey filaments compared with saline control (p < 0.05) for a period of at least 6 h. Wounds healed normally with no signs of infection, redness or swelling.
Conclusions
We propose that the pig model of incisional pain can provide an appropriate translational model for validating new topical and localized treatments for POP in humans.
D. Castel, E. Willentz, O. Donnor, O Brenner, S. Meilin. Characterization of a porcine model of post-operative pain. European J Pain, Sept 2013,
Read More
Topics:
Pain
Sciatic Nerve Block: Rapid model for evaluating nerve blocking agents or drugs designed to reverse local analgesia.
Nerve blocking agents are often used to replace general anesthesia in certain surgical procedures or for providing pain relieve in the post-operative period where the nerve block tends to provide more effect pain control and reduce opioid-related side effects. The rapid preclinical model of sciatic nerve block allows the screening of new nerve blocking agents or drugs designed to reverse the local analgesia. The surgical method employed with the nerve block model can also be applied to other models of pain where direct dosing to the sciatic nerve is desired rather than systemic administration.
The nerve block model is based on the administration of nerve blocking agents directly to the saphenous and sciatic nerves. Local injection is performed to the adductor canal as well as to the sciatic notch. Following the administration of nerve blocking agents, thermal hyperalgesia is tested for a duration relevant to the nerve blocking agent or drug being tested.
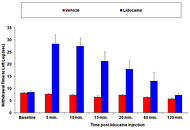
Review the surgical procedure and data for the model of nervel block.
About MD Biosciences
MD Biosciences is a Preclinical Contract Research Organization (CRO) providing services and products for biotech/pharmaceutical, medical device and animal health and companies engaged in inflammations & neurology research. With specialized laboratories located in Minnesota, Glasgow, and Israel, our panel of internationally recognized experts provides in-depth expertise and technologies to overcome challenges and provide total solutions to the drug discovery market.
--------
The information in this press release should be considered accurate only as of the date of the release. MDB has no intention of updating and specifically disclaims any duty to update the information in these press releases.
Read More
Topics:
Pain
 With a multifaceted approach to assessment of neurological diseases, specializations include pain-related work, neurodegenerative diseases including Parkinson's, neuro-inflammatory diseases such as Multiple Sclerosis (MS), and capabilities in stroke and cerebral ischemia. Preclinical models are complimented by in house histology, biomarker analysis and electrophysiology capabilities. Finally, our unique porcine models share a high degree of similarity to humans, thus offering greater clinical efficacy that serve to augment and accelerate your program towards their next objectives and milestones.
With a multifaceted approach to assessment of neurological diseases, specializations include pain-related work, neurodegenerative diseases including Parkinson's, neuro-inflammatory diseases such as Multiple Sclerosis (MS), and capabilities in stroke and cerebral ischemia. Preclinical models are complimented by in house histology, biomarker analysis and electrophysiology capabilities. Finally, our unique porcine models share a high degree of similarity to humans, thus offering greater clinical efficacy that serve to augment and accelerate your program towards their next objectives and milestones.








 MD Biosciences article Characterization of a porcine model of post-operative pain (POP)
MD Biosciences article Characterization of a porcine model of post-operative pain (POP)






